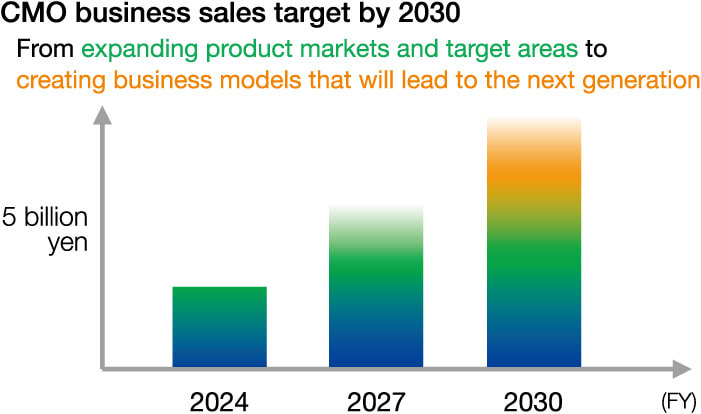
CMO Buisiness
Contributing to the global pharmaceutical supply system
The pharmaceutical contract manufacturing organization (CMO) market continues to grow globally against the backdrop of efforts to control medical costs and diversify drug discovery modalities. In particular, for rare diseases and regenerative medicine, there is demand for small lots and a wide variety of products, and companies with flexible inspection and packaging systems are becoming increasingly important.
To meet these needs, we have been using our knowledge of packaging technology and quality control to work to eliminate the drug lag and drug loss issues facing pharmaceutical companies. In FY2024, the CMO business achieved sales of over 3 billion yen, marking the first step towards solid growth.
Based on this track record, we will expand our inspection and packaging know-how to overseas markets in the future and play an important role in the global pharmaceutical supply system.
The key to growth: How to ensure trust and speed
"Gaining trust" and "speedy response" are essential for the growth of the CMO market. When receiving orders from pharmaceutical companies, we are required to have a quality assurance system that complies with GMP, a track record of handling inspections, and the ability to provide a stable supply.
In the future, in anticipation of foreign pharmaceutical companies and bio ventures entering the Japanese market, we will increase our investment in human resources with practical experience in the global pharmaceutical industry. As part of this, we will continue to hire personnel with experience in the pharmaceutical and CRO* industries and build collaborative systems with customers from the drug development stage. In addition, we employ specialists who can comply with international standards such as FDA and PIC/S as heads of manufacturing and quality departments.
*CRO (Contract Research Organization): An organization that undertakes the development of pharmaceuticals and medical devices.
Growth strategy: Combining alliances and M&A
Our CMO business is strategically expanding its business through both external alliances and M&A while establishing a competitive edge in the niche area specializing in inspection and packaging.
In terms of external collaboration, we have built a system through which we can make proposals to pharmaceutical companies from the early development stages through collaboration with CROs. We are also proactively developing alliances with external partners that comply with GDP, enabling us to provide a consistent one-stop service from "inspection and packaging → storage → distribution."
In particular, collaboration with pharmaceutical wholesalers and logistics companies will lead to the strengthening of supply infrastructure to meet the needs of the global market, contributing to the optimization of the entire value chain and the creation of added value.
Through M&A, we are gradually acquiring companies that have strengths in secondary packaging for small molecule drugs and biopharmaceuticals. By incorporating workforce, facilities, and know-how that can be put to immediate use, we aim to strengthen the foundation for sustainable growth.

Overseas expansion and preparations for the future
To respond to expanding demand in overseas markets, we will continue to expand our business internationally, with a view to forming technical alliances with local pharmaceutical companies and establishing bases through joint ventures with companies that have pharmaceutical distribution networks.
Moreover, in collaboration with our company's line engineering division, we have begun developing next-generation inspection equipment that uses AI technology. We will build a global inspection and packaging model that combines quality, speed, and efficiency.
Our CMO business will continue to take on new challenges and grow, aiming to become an indispensable part of healthcare and society.